
SAPONIFICATION
Table of Contents
- What is Saponification?
- Saponification Soap Making
- Temperatures
- Using Lye & Potash
- Trace
- Gel Phase
- Curing
- Insulate or Refrigerate?
- Cosmetic Concerns
- Testing PH
- SAP Chart
- Fatty Acid Profile
- Oil Properties Chart
- When Things Go Wrong
- Safety & Precautions
- Applications
- Soaps: Soft vs. Hard
- Lithium & Amide Soaps
- Fire Extinguishers
- Oil Paints
- Grave Wax
- See Also
- References
- Further Reading
- External Links

SAPONIFICATION
The Art, Chemistry & Science of Soap-Making
What is Saponification?
Saponification is a chemical reaction between any fat and an alkali, such as NaOH (sodium hydroxide). The creation of soap is the result of saponification. Very merely, making soap by mixing animal or vegetable oils or fats with lye that has been hydrated (dissolved in water). The chemical reaction which occurs results in a marvelous new substance – soap!
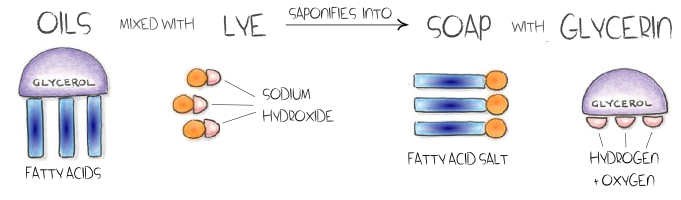
Saponification can often occur naturally, such as in corpses, antique oil paintings, and even when it rains on your grill ashes. Due to incredible advances in technology in the 18th century, saponification can be refined and controlled, allowing artisans to make beautiful handmade soaps. Artisan soap makers typically produce soaps that retain their glycerol (glycerin), whereas commercial production is usually processed to remove the glycerin.


Applications of Saponification
Soft versus hard soap
Depending on the nature of the alkali used in their production, soaps have distinct properties. Sodium hydroxide (NaOH) gives “hard soap”; hard solvents can also be used in water containing Mg, Cl, and Ca salts. By contrast, when using potassium hydroxide (KOH), a soft soap is formed.
Hard soap or curd soap is a kind of soap; examples are Aleppo soap, Castile soap, and Marseille soap or Savon de Marseille. During the preparation of the soap, common salt (sodium chloride) is added to the liquid soap mass. The addition of the sodium chloride leads to the soap mass separating from glycerin, resulting in a harder soap.
Despite the removal of glycerin, these soaps are quite conditioning. They are constituting a minimum of 72% olive oil for Marseille soap, 100% olive oil for Castile soap, and 80/20 olive oil/bay laurel berry oil for Aleppo soap.
Lithium Soaps
 >Lithium derivatives of 12-hydroxy stearate and several other carboxylic acids are essential constituents of lubricating greases. In lithium-based lubricants, lithium carboxylates are thickeners. “Complex soaps” are also ordinary, these being combinations of metallic soaps, such as lithium and calcium soaps.
>Lithium derivatives of 12-hydroxy stearate and several other carboxylic acids are essential constituents of lubricating greases. In lithium-based lubricants, lithium carboxylates are thickeners. “Complex soaps” are also ordinary, these being combinations of metallic soaps, such as lithium and calcium soaps.
Lithium soaps can be made like saponification of triglycerides. However, instead of sodium hydroxide, the fatty acids are treated with lithium hydroxide or lithium carbonate to form lithium salts of fatty acids. The lithium salts are colorless solids that melt near 200 °C (390 °F).
Fire extinguishers & Saponification
 Fires involving cooking fats and oils (classified as class K (US) or F (Australia/Europe/Asia)) burn hotter than flammable liquids, rendering a standard class B extinguisher ineffective. Flammable liquids have flash points under 37 degrees Celsius. Cooking oil is a combustible liquid, since it has a flash point over 37 degrees Celsius. Such fires should be extinguished with a wet chemical extinguisher. Extinguishers of this type are designed to extinguish cooking fats and oils through saponification. The extinguishing agent rapidly converts the burning substance to a non-combustible soap. This process is endothermic, meaning that it absorbs thermal energy
Fires involving cooking fats and oils (classified as class K (US) or F (Australia/Europe/Asia)) burn hotter than flammable liquids, rendering a standard class B extinguisher ineffective. Flammable liquids have flash points under 37 degrees Celsius. Cooking oil is a combustible liquid, since it has a flash point over 37 degrees Celsius. Such fires should be extinguished with a wet chemical extinguisher. Extinguishers of this type are designed to extinguish cooking fats and oils through saponification. The extinguishing agent rapidly converts the burning substance to a non-combustible soap. This process is endothermic, meaning that it absorbs thermal energy
Saponification of Oil Paints

Detail of Madame X (Madame Pierre Gautreau), John Singer Sargent, 1884, showing saponification in the black dress.
Saponification can occur in oil paintings over time, causing visible damage and deformation. The ground layer or paint layers of oil paintings commonly contain heavy metals in pigments such as lead white, red lead, or zinc white. If those heavy metals react with free fatty acids in the oil medium that binds the pigments together, soaps may form in a paint layer that can then migrate outward to the painting’s surface.
Centuries to Saponify
Saponification in oil paintings was described as early as 1912. It is believed to be widespread, having been observed in many works dating from the fifteenth through the twentieth centuries, works of different geographic origin, and works painted on various supports, such as canvas, paper, wood, and copper. Chemical analysis may reveal saponification occurring in a painting’s deeper layers before any signs are visible on the surface, even in paintings centuries old.
The saponified regions may deform the painting’s surface through the formation of visible lumps or protrusions that can scatter light. These soap lumps may be prominent only on certain regions of the painting rather than throughout. In John Singer Sargent’s famous Portrait of Madame X, for example, the lumps only appear on the blackest areas, which may be because of the artist’s use of more medium in those areas to compensate for the tendency of black pigments to soak it up.The process can also form chalky white deposits on a painting’s surface, a deformation often described as “blooming” or “efflorescence,” and may also contribute to the increased transparency of certain paint layers within an oil painting over time.
Restoration Method
The process is still not fully understood. Saponification does not occur in all oil paintings containing the right materials. It is not yet known what triggers the process, what makes it worse, or whether it can be halted. At present, retouching is the only known restoration method.
Saponification of Corpses
Adipocere, also known as corpse wax or the fat of graveyards, is a product of decomposition that turns body fat into a soap-like substance. Corpse wax forms through a process called saponification and tends to develop when body fat is exposed to anaerobic bacteria in a warm, damp, alkaline environment, either in soil or water. Grave wax has a soft, greasy gray appearance when it starts to form, and as it ages the wax hardens and turns brittle. Saponification will stop the decay process in its tracks by encasing the body in this waxy material, turning it into a “soap mummy.”

Soapman: Corpse Turned Completely Into Soap
Affiliate Disclosure: I am grateful to be of service and bring you content free of charge. In order to do this, please note that when you click links and purchase items, in most (not all) cases I will receive a referral commission. Your support in purchasing through these links enables me to keep Par Par Academy of Soaps & Cosmetics free, and empower more people worldwide to make soap safely and easily. Thank you!